Suntan Aluminum Periodic Table
Basic Information:
Atomic Number: 13
Atomic Mass: 26.981539 amu
Melting Point: 660.37℃ (933.52 K, 1220.666 °F)
Boiling Point: 2467.0℃ (2740.15 K, 4472.6 °F)
Number of Protons/Electrons: 13
Number of Neutrons: 14
Classification: Other Metals
Crystal Structure: Cubic
Density @ 293 K: 2.702 g/cm3
Color: Silver
British Spelling: Aluminium
IUPAC Spelling: Aluminium
Atomic Structure:
| Number of Energy Levels: 3 | 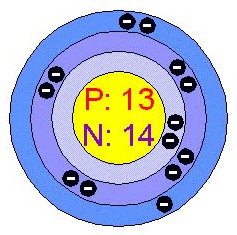 |
| First Energy Level: 2 | |
| Second Energy Level: 8 | |
| Third Energy Level: 3 |
Isotopes:
| Isotope | Half Life |
| AI-26 | 730000.0 years |
| AI-27 | Stable |
| AI-28 | 2.3 minutes |
Facts:
Date of Discovery: 1825
Discoverer: Hans Christian Oersted
Name Origin: From the Latin word alumen
Uses: airplanes, soda cans
Obtained From: bauxite